Berikut adalah tabel unsur kimia yang disusun berdasarkan nomor atom dan kode warna menurut tipe unsur. Setiap unsur ditampilkan informasi mengenai nama unsur, lambang unsur, golongan dan periode, massa atom (atau isotop yang paling stabil), massa jenis, titik lebur, titik didih dan penemunya. The Manitoba Archival Information Information Network (MAIN) is powered by Access to Memory (AtoM) software. The AMA provides training sessions where members are instructed on how to enter archival descriptions, authority records, and uploading digital objects to MAIN.
| LESSON 1: Basic Chemistry |
Atoms & Ions | Chemical Bonding | Water | Acid & Bases | Organic Compounds | Carbohydrates | Lipids | Proteins | Enzymes | Nucleic Acids | ATP |
|
ATOMS AND IONS
Atoms
Atoms are the basic unit of chemistry. They consist of 3 smaller things:
- Protons - these are positively charged (+)
- Electrons - these are negatively charged (-)
- Neutrons - these have no charge
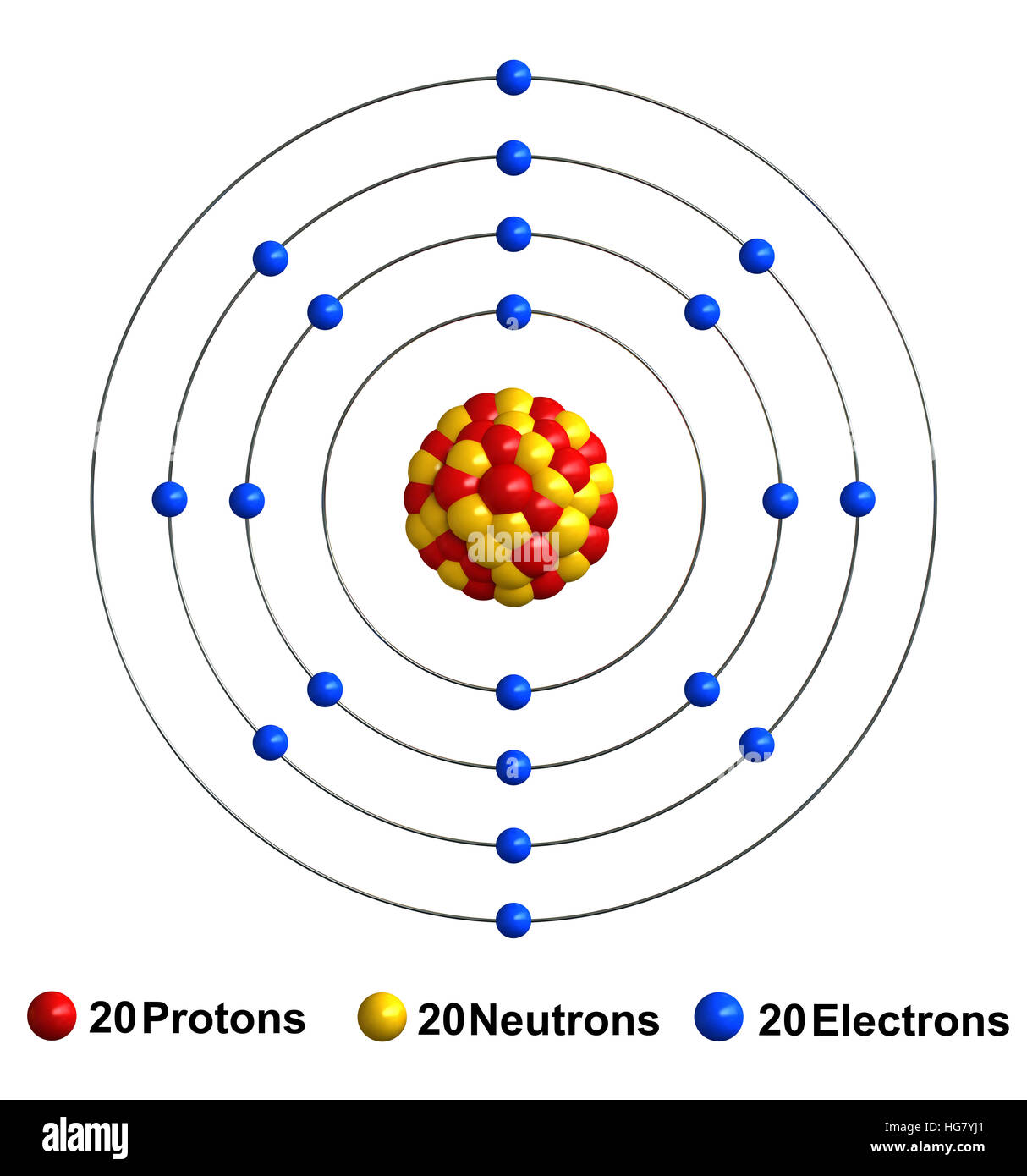
These 3 smaller particles are arranged in a particular way. In the center is the Nucleus where you find the positive Protons and neutral Neutrons.
In orbit around the nucleus are the Electrons. These are found in a series of orbits (depending on the atom) with differing numbers of electrons as seen below.
Interaction of Atoms
It's the electrons in orbit around the nucleus that allow one atom to interact with other atoms so they can be linked together.
For example, H2O consists of an Oxygen atom linked to 2 Hydrogen atoms. The linkage or interaction between the electrons of the Hydrogen and Oxygen atoms is called a Chemical Bond. More on these later.
Atoms in the Human Body
The human body is made up of a couple dollars worth of chemicals.
The 12 most useful atoms for you to know about are listed below:
Ions
Sometimes atoms gain or lose electrons. The atom then loses or gains a 'negative' charge. These atoms are then called ions.
- Positive Ion - Occurs when an atom loses an electron (negative charge) it has more protons than electrons.
- Negative Ion - Occurs when an atom gains an electron (negative charge) it will have more electrons than protons.
The following image shows Na losing an electron and Cl gaining an electron
Ca Atomic Number Of Protons
- Thus the Na becomes Na+
- The Cl becomes Cl-
Ca Atomic Structure
| Here are some examples of common ions: | |
| Na+ | Sodium |
| K+ | Potassium |
| Cl- | Chloride |
| Ca+ | Calcium |
| Fe+ | Iron |
| P- | Phosphorous |
Home |
